Cyanohydrin reaction
Template:Short description Template:Use dmy dates Template:Reactionbox
In organic chemistry, a cyanohydrin reaction is an organic reaction in which an aldehyde (Template:Chem2) or ketone (Template:Chem2) reacts with a cyanide anion (Template:Chem2) or a nitrile (Template:Chem2) to form a cyanohydrin (Template:Chem2). For example:
This nucleophilic addition is a reversible reaction but with aliphatic carbonyl compounds equilibrium is in favor of the reaction products. The cyanide source can be potassium cyanide (KCN), sodium cyanide (NaCN) or trimethylsilyl cyanide (Template:Chem2). With aromatic aldehydes such as benzaldehyde, the benzoin condensation is a competing reaction. The reaction is used in carbohydrate chemistry as a chain extension method for example that of D-xylose.
Examples


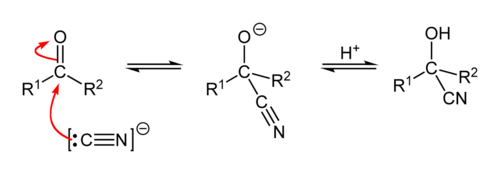
Reaction mechanism
Asymmetric synthesis
The asymmetric cyanohydrin reaction of benzaldehyde with trimethylsilylcyanide is made possible by employment of (R)-Binol[1] at 1–10% catalyst loading. This ligand firsts reacts with a lithium alkoxy compound to form a lithium binaphtholate Complex.

The chemist Urech in 1872 was the first to synthesize cyanohydrins from ketones with alkali cyanides and acetic acid[2] and therefore this reaction also goes by the name of Urech cyanohydrin method.
References
External links
- Cyanohydrin reaction of formaldehyde to hydroxyacetonitrile or glycolonitrile with sodium cyanide in Organic Syntheses Coll. Vol. 2, p. 387; Vol. 13, p. 56 Article
- Cyanohydrin reaction of formaldehyde with potassium cyanide Organic Syntheses Coll. Vol. 3, p. 436; Vol. 27, p. 41 Article
- Cyanohydrin reaction of acetophenone with potassium cyanide Organic Syntheses Coll. Vol. 4, p. 58; Vol. 33, p. 7 Article
- Cyanohydrin reaction of D-xylose with potassium cyanide Organic Syntheses Coll. Vol. 4, p. 506; Vol. 36, p. 38 Article
- Cyanohydrin reaction of acetone with potassium cyanide Organic Syntheses Coll. Vol. 2, p. 7; Vol. 15, p. 1 Article
- Cyanohydrin reaction of benzoquinone with trimethylsilylcyanide Organic Syntheses Coll. Vol. 7, p. 517; Vol. 60, p. 126 Article