Ammonia (data page)
Jump to navigation
Jump to search
Template:Short description Template:Use dmy dates
This page provides supplementary chemical data on ammonia.
Structure and properties
| Template:Chembox header | Molecular structure | |
|---|---|
| Point group | C3v |
| Bond length | 101.2 pm (N–H)[1] |
| Bond angle | 106.7° (H–N–H)[1] |
| Bond strength | 435 kJ/mol (H–NH2) |
| Template:Chembox header | Crystal data | |
| Crystal structure | ? |
| Template:Chembox header | Properties | |
| Dipole moment | 1.46 D |
| Dielectric constant | 22 ε0 at 239 K |
| Magnetic susceptibility | diamagnetic |
| Acidity of NH4+ (pKa) | 9.25 |
Thermodynamic properties
I. cubic, II. hcp, III. fcc, IV. orthorhombic
| Template:Chembox header | Phase behavior | |
|---|---|
| Triple point | 195.4 K (−77.75 °C), 6.060 kPa |
| Critical point | 405.5 K (132.3 °C), 11.300 MPa |
| Std enthalpy change of fusion, ΔfusH |
+5.653 kJ/mol |
| Std entropy change of fusion, ΔfusS |
+28.93 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
+23.35 kJ/mol at BP of −33.4 °C |
| Std entropy change of vaporization, ΔvapS |
+97.41 J/(mol·K) at BP of −33.4 °C |
| Template:Chembox header | Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Template:Chembox header | Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−80.882 ± 0.053 kJ/mol[2] |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | 80.80 J/(mol K) |
| Template:Chembox header | Gas properties | |
| Std enthalpy change of formation, ΔfH |
−45.556 ± 0.029 kJ/mol[3] |
| Std Gibbs free energy change of formation, ΔfG |
−16.6 kJ/mol |
| Standard molar entropy, S |
192.77 J/(mol K) |
| Heat capacity, cp | 35.06 J/(mol K) |
| Heat capacity ratio, γ at 15 °C |
1.310 |
| van der Waals' constants | a = 422.5 L2 kPa/mol2 b = 0.03707 L/mol |
Vapor–liquid equilibrium data
| Template:Chembox header | P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15600 | 30400 | 45600 |
| Template:Chembox header | T in °C | −109.1(s) | −91.9(s) | −79.2(s) | −68.4 | −45.4 | −33.6 | −18.7 | 4.7 | 25.7 | 50.1 | 78.9 | 98.3 |
Table data (above) obtained from CRC Handbook of Chemistry and Physics 44th ed. The (s) notation indicates equilibrium temperature of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid.
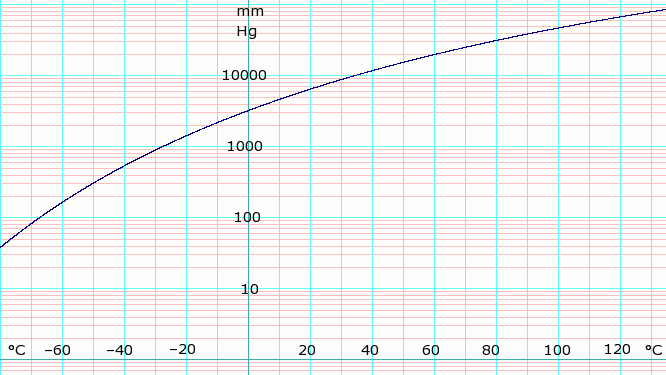
Vapor-pressure formula for ammonia:[4]
- log10P = A – B / (T − C),
where P is pressure in kPa, and T is temperature in kelvins;
- A = 6.67956, B = 1002.711, C = 25.215 for T = 190 K through 333 K.
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Heat capacity of liquid and vapor
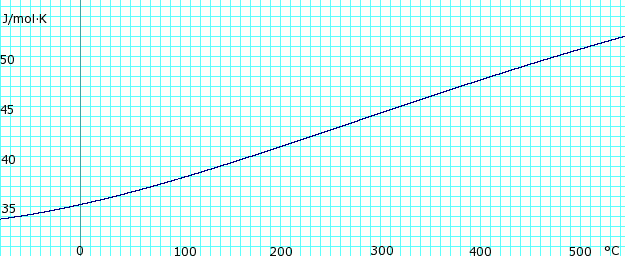 |
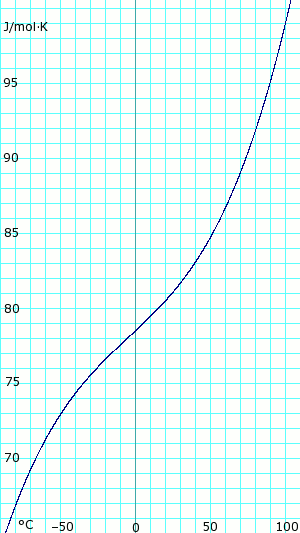 |
Spectral data
| Template:Chembox header | UV-Vis | |
|---|---|
| λmax | None nm |
| Extinction coefficient, ε | None |
| Template:Chembox header | IR | |
| Major absorption bands | 3444, 3337, 1627, 950 cm−1 |
| Template:Chembox header | NMR | |
| Proton NMR | |
| Carbon-13 NMR | None – no carbons |
| Other NMR data | |
| Template:Chembox header | MS | |
| Masses of main fragments |
17 (100%) 16(80%) 15(9%) |
Regulatory data
| Template:Chembox header | Regulatory data | |
|---|---|
| EINECS number | 231-635-3 (gas) 215-647-6 (soln.) |
| EU index number | 007-001-00-5 (gas) 007-001-01-2 (soln.) |
| PEL-TWA (OSHA) | 50 ppm (35 mg/m3) |
| IDLH (NIOSH) | 300 ppm |
| Flash point | 11 °C |
| Autoignition temperature | 651 °C |
| Explosive limits | 15–28% |
| RTECS # | BO0875000 |
Safety data sheet
The handling of this chemical may incur notable safety precautions... It is highly recommend that you seek the Safety Data Sheet (SDS) for this chemical from a reliable source and follow its directions.
- SIRI
- Science Stuff (Ammonia Solution)
References
- ↑ 1.0 1.1 CRC Handbook of Chemistry and Physics, 94th ed. http://www.hbcpnetbase.com Template:Webarchive. Page 9-26. Retrieved 18 June 2013.
- ↑ "Ammonia - NH3 (aq, undissoc)" Active Thermochemical Tables v1.130. https://atct.anl.gov/Thermochemical%20Data/version%201.130/species/?species_number=1041
- ↑ "Ammonia - NH3(g)" Active Thermochemical Tables v1.130. https://atct.anl.gov/Thermochemical%20Data/version%201.130/species/?species_number=35
- ↑ Lange's Handbook of Chemistry, 10th ed. page 1436.
- ↑ Lange's Handbook of Chemistry, 10th ed. page 1451 and 1468.
- ↑ Template:Cite book
- ↑ Perman, Jour. Chem. Soc. 83 1168 (1903).
- ↑ 8.0 8.1 Template:Cite web
Template:Chemical data page general note
External links
- Phase diagram for ammonia
- IR spectrum (from NIST)