List of psychoactive plants
Template:Short description Template:Multiple issues Template:Main

This is a list of plant species that, when consumed by humans, are known or suspected to produce psychoactive effects: changes in nervous system function that alter perception, mood, consciousness, cognition or behavior. Many of these plants are used intentionally as psychoactive drugs, for medicinal, religious, and/or recreational purposes. Some have been used ritually as entheogens for millennia.[1][2]
The plants are listed according to the specific psychoactive chemical substances they contain; many contain multiple known psychoactive compounds.
Cannabinoids

Template:Main Species of the genus Cannabis, known colloquially as marijuana, including Cannabis sativa and Cannabis indica, is a popular psychoactive plant that is often used medically and recreationally. The principal psychoactive substance in Cannabis, tetrahydrocannabinol (THC), contains no nitrogen, unlike many (but not all) other psychoactive substancesTemplate:Efn and is not an indole, tryptamine, phenethylamine, anticholinergic (deliriant) or dissociative drug. THC is just one of more than 100 identified cannabinoid compounds in Cannabis, which also include cannabinol (CBN) and cannabidiol (CBD).
Cannabis plants vary widely, with different strains producing dynamic balances of cannabinoids (THC, CBD, etc.) and yielding markedly different effects. Popular strains are often hybrids of C. sativa and C. indica.
The medicinal effects of cannabis are widely studied, and are active topics of research both at universities and private research firms. Many jurisdictions have laws regulating or prohibiting the cultivation, sale and/or use of medical and recreational cannabis.Template:Citation needed
Tryptamines





Many of the psychedelic plants contain dimethyltryptamine (DMT), or other tryptamines, which are either snorted (Virola, Yopo snuffs), vaporized, or drunk with MAOIs (Ayahuasca). It cannot simply be eaten as it is not orally active without an MAOI and it needs to be extremely concentrated to be vaporized.
"Species, Alkaloid content, where given, refers to dried material"
- Fittonia albivenisTemplate:Citation needed, a common ornamental plant from South America.
- Acer saccharinum (silver maple) was found to contain the indole alkaloid gramine (not active and extremely toxic) 0.05% in the leaves, so it is possible that other members of this plant family contain active compounds.[3]
- Delosperma acuminatum, DMT, 5-MeO-DMT[4]Template:Unreliable source?
- Delosperma cooperi, DMT, 5-MeO-DMT[4]
- Delosperma ecklonis, DMT[4]
- Delosperma esterhuyseniae, DMT[4]
- Delosperma hallii, 5-MeO-DMT[4]
- Delosperma harazianum, DMT, 5-MeO-DMT[4]
- Delosperma harazianum
Shibam, DMT[4]
- Delosperma harazianum
- Delosperma hirtum, DMT[4]
- Delosperma hallii
aff. litorale
- Delosperma hallii
- Delosperma lydenbergense, DMT, 5-MeO-DMT[4]
- Delosperma nubigenum, 5-MeO-DMT[4]
- Delosperma pageanum, DMT, 5-MeO-DMT[4]
- Delosperma pergamentaceum, Traces of DMT[4]
- Delosperma tradescantioides, DMT[4]
- Prestonia amazonica: DMT[5]
- Voacanga africana: Up to 10% Iboga alkaloids[6]
Fabaceae (Leguminosae)






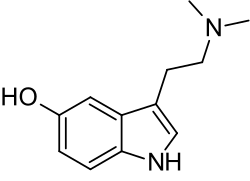






- Acacia acuminata, Up to 1.5% alkaloids, mainly consisting of dimethyltryptamine in bark & leaf[8]Template:Unreliable source? Also, harman, tryptamine, NMT, other alkaloids in leaf.Template:Citation needed
- Acacia alpina, Active principles in leaf[9]Template:Unreliable source?
- Acaciella angustissima, β-methyl-phenethylamine,[10] NMT and DMT in leaf (1.1-10.2 ppm)[11]
- Vachellia aroma, Tryptamine alkaloids.[12] Significant amount of tryptamine in the seeds.[13]
- Acacia auriculiformis, 5-MeO-DMT in stem bark[14]
- Acacia baileyana, 0.02% tryptamine and β-carbolines, in the leaf, Tetrahydroharman[15]
- Acacia beauverdiana, Psychoactive[16] Ash used in Pituri.[17]
- Senegalia berlandieri, DMT, phenethylamine, mescaline, nicotine[18]
- Senegalia catechu, DMT and other tryptamines in leaf, barkTemplate:Citation needed
- Vachellia caven, Psychoactive[6]
- Senegalia chundra, DMT and other tryptamines in leaf, barkTemplate:Citation needed
- Acacia colei, DMT[19]
- Acacia complanata, 0.3% alkaloids in leaf and stem, almost all N-methyl-tetrahydroharman, with traces of tetrahydroharman, some of tryptamine[20][21][22]
- Acacia confusa, DMT & NMT in leaf, stem & bark 0.04% NMT and 0.02% DMT in stem.[9] Also N,N-dimethyltryptamine N-oxide[23]
- Vachellia cornigera, Psychoactive,[6] Tryptamines[24] DMT according to C. Rastch.
- Acacia cultriformis, Tryptamine, in the leaf, stem[9] and seeds.[13] Phenethylamine in leaf and seeds[13]
- Acacia cuthbertsonii, Psychoactive[6]
- Acacia decurrens, Psychoactive,[6] but less than 0.02% alkaloids[15]
- Acacia delibrata, Psychoactive[16]
- Acacia falcata, Psychoactive,[16] but less than 0.02% alkaloids[15]
- Vachellia farnesiana, Traces of 5-MeO-DMT[25] in fruit. β-methyl-phenethylamine, flower.[26] Ether extracts about 2–6% of the dried leaf mass.[27] Alkaloids are present in the bark[28] and leaves.[29]
- Acacia flavescens, Strongly Psychoactive, BarkTemplate:Citation needed
- Acacia floribunda, Tryptamine, phenethylamine,[30] in flowers[13] other tryptamines,[31] DMT,tryptamine,NMT 0.3–0.4% phyllodes.[32]
- Acacia georginae, Psychoactive,[6] plus deadly toxins
- Vachellia horrida, Psychoactive[6]
- Acacia implexa, Psychoactive[33]
- Mimosa jurema, DMT,[6] NMTTemplate:Citation needed
- Vachellia karroo, PsychoactiveTemplate:Citation needed
- Senegalia laeta, DMT, in the leaf[9]
- Acacia longifolia, 0.2% tryptamine in bark, leaves, some in flowers, phenylethylamine in flowers,[30] 0.2% DMT in plant.[34] Histamine alkaloids.[15]
- Acacia sophorae, Tryptamine in leaves, bark[13]
- Acacia macradenia, Tryptamine[13]
- Acacia maidenii, 0.6% NMT and DMT in about a 2:3 ratio in the stem bark, both present in leaves[9]
- Acacia mangium, Psychoactive[6]
- Acacia melanoxylon, DMT, in the bark and leaf,[35] but less than 0.02% total alkaloids[15]
- Senegalia mellifera, DMT, in the leaf[9]
- Vachellia nilotica, DMT, in the leaf[9]
- Vachellian ilotica subsp. adstringens, Psychoactive, DMT in the leafTemplate:Citation needed
- Acacia neurophylla DMT in bark, Harman in leaf.[36]
- Acacia obtusifolia, Tryptamine, DMT, NMT, other tryptamines,[37] 0.4–0.5% in dried bark,0.15–0.2% in leaf, 0.07% in branch tips.[38]
- Vachellia oerfota, Less than 0.1% DMT in leaf,[39] NMT
- Acacia penninervis, Psychoactive[16]
- Acacia phlebophylla, 0.3% DMT in leaf, NMT[9]
- Acacia podalyriifolia, Tryptamine in the leaf,[9] 0.5% to 2% DMT in fresh bark, phenethylamine, trace amounts.[30] Although this species is claimed to contain 0.5% to 2% DMT in fresh bark the reference for this is invalid as there is no reference to Acacia Podalyriffolia anywhere in the reference article. Additionally, well known and proven extraction techniques for DMT have failed to produce any DMT or alkaloids from fresh bark or the leaves on multiple sample taken at various seasons. Should DMT actually exist in this species of Acacia then it exists in extremely small amounts and have failed to produce any alkaloids with Acid/Base extraction techniques using HCl/Na(OH)2. On the same note, more academic research is definitely required into the DMT content of this and other Australian Acacia species with proper chemical analysis of sample.Template:Citation needed
- Senegalia polyacantha, DMT in leaf[9] and other tryptamines in leaf, bark
- Senegalia polyacantha ssp. campylacantha, Less than 0.2% DMT in leaf, NMT; DMT and other tryptamines in leaf, bark[40]
- Senegalia rigidula: Phenethylamine, tryptamine, tyramine, and β-Methylphenethylamine.[41]
- Acacia sassa, Psychoactive[6]
- Vachellia schaffneri, β-methyl-phenethylamine, Phenethylamine[42]
- Senegalia senegal, Less than 0.1% DMT in leaf,[9] NMT, other tryptamines. DMT in plant,[26] DMT in bark.[13]
- Vachellia seyal, DMT, in the leaf.[9] Ether extracts about 1–7% of the dried leaf mass.[27]
- Vachellia sieberiana, DMT, in the leaf[9]
- Acacia simplex, DMT and NMT, in the leaf, stem and trunk bark, 0.81% DMT in bark, MMT[9][43]
- Vachellia tortilis, DMT, NMT, and other tryptamines[33]
- Acacia vestita, Tryptamine, in the leaf and stem,[9] but less than 0.02% total alkaloids[15]
- Acacia victoriae, tryptamines, 5-MeO-alkyltryptamine[13]
- List of acacia species having little or no alkaloids in the material sampled:[15]
(0% C 0.02%, Concentration of alkaloids) - Pseudalbizzia inundata leaves contain DMT.[6]
- Anadenanthera colubrina, Bufotenin, Beans,[44][45] Bufotenin oxide, Beans,[44] N,N-Dimethyltryptamine, Beans,[44][45] pods,[44]
- Anadenanthera colubrina var. cebil – Bufotenin and Dimethyltryptamine have been isolated from the seeds and seed pods, 5-MeO-DMT from the bark of the stems.[46] The seeds were found to contain 12.4% bufotenine, 0.06% 5-MeO-DMT and 0.06% DMT.[47]
- Anadenanthera peregrina,
1,2,3,4-Tetrahydro-6-methoxy-2,9-dimethyl-beta-carboline, Plant,[48] 1,2,3,4-Tetrahydro-6-methoxy-2-methyl-beta-carboline, Plant,[45] 5-Methoxy-N,N-dimethyltryptamine, Bark,[45] 5-Methoxy-N-methyltryptamine, Bark,[45] Bufotenin, plant,[45] beans,[44] Bufotenin N-oxide, Fruit,[45] beans,[44] N,N-Dimethyltryptamine-oxide, Fruit[45][49]
- Anadenanthera peregrina var. peregrina, Bufotenine is in the seeds.[50]
- Desmanthus illinoensis, 0–0.34% DMT in root bark, highly variable.[51] Also NMT, N-hydroxy-N-methyltryptamine, 2-hydroxy-N-methyltryptamine, and gramine (toxic).[52]
- Desmanthus leptolobus, 0.14% DMT in root bark, more reliable than D. illinoensis[51]
- Desmodium caudatum[53] (syn. Ohwia caudata), Roots: 0.087% DMT,
- Codariocalyx motorius(syn. Desmodium gyrans), DMT, 5-MeO-DMT, leaves, rootsTemplate:Citation needed
- Desmodium racemosum, 5-MeO-DMT[6]
- Desmodium triflorum, 0.0004% DMT-N-oxide, roots,[54] less in stems[54] and trace in leaves.[54]
- Lespedeza capitata[6]
- Lespedeza bicolor, DMT, Lespedamine,[55] and 5-MeO-DMT in leaves and roots[56]
- Lespedeza bicolor var. japonica, DMT, 5-MeO-DMT[6] in leaves and root bark
- Mimosa ophthalmocentra, Dried root: DMT 1.6%, NMT 0.0012% and hordenine 0.0065%[57]
- Mimosa scabrella, tryptamine, NMT, DMT and N-methyltetrahydrocarboline in bark[58]
- Mimosa somnians, tryptamines and MMTTemplate:Citation needed
- Mimosa tenuiflora (syn. "Mimosa hostilis"), 0.31-0.57% DMT (dry root bark).[59]
- Mimosa verrucosa, DMT[60] in root bark
- Mucuna pruriens, the seeds of the plant contain about 3.1–6.1% Template:Nowrap.[61]
- Petalostylis casseoides, 0.4–0.5% tryptamine, DMT, etc. in leaves and stems[56]
- Petalostylis labicheoides var. casseoides, DMT[6] in leaves and stems; 0.4–0.5% alkaloids in leaves and stems;[62] Tryptamines in leaves and stems, MAO's up to 0.5%[63]Template:Unreliable source?
- Phyllodium pulchellum(syn. Desmodium pulchellum), DMT;[6] 0.2% 5-MeO-DMT, small quantities of DMT[56] DMT (dominates in seedlings and young plants), 5-MeO-DMT (dominates in mature plant), whole plant, roots, stems, leaves, flowers;
- Erythrina flabelliformis, other Erythrina species, seeds contain the alkaloids erysodin and erysovin[64]Template:Better source needed
- Zornia latifolia, the flavones genistein, apigenin and syzalterin may explain the cannabis-like effects[65][66]
- Diplopterys cabrerana: McKenna et al. (1984) assayed and found the leaves contain 0.17% DMT[68]
- Horsfieldia superba: 5-MeO-DMT,[56] Horsfiline,[69] and beta-carbolines[62]
- Iryanthera macrophylla: 5-MeO-DMT in bark;[56]
- Iryanthera ulei: 5-MeO-DMT in bark[6]
- Osteophloem platyspermum: DMT, 5-MeO-DMT in bark
- Virola calophylla, Leaves 0.149% DMT, leaves 0.006% MMT 5-MeO-DMT in bark[70]
- Virola calophylloidea, DMT, 5-MeO-DMT[6]
- Virola carinata, DMT in leaves; DMT, 5-MeO-DMT[6]
- Virola cuspidata, DMT[63]
- Virola divergens, DMT[6] in leaves
- Virola elongata(syn. Virola theiodora), DMT, 5-MeO-DMT[6] in bark, roots, leaves and flowers
- Virola melinonii, DMT in bark; DMT, 5-MeO-DMT[6]
- Virola multinervia, DMT, 5-MeO-DMT[6] in bark and roots
- Virola pavonis, DMT[6] in leaves
- Virola peruviana, DMT, 5-MeO-DMT;[6] 5-MeO-DMT, traces of DMT and 5-MeO-tryptamine in bark
- Virola rufula, Alkaloids in bark and root, 95% of which is MeO-DMT[71] 0.190% 5-MeO-DMT in bark, 0.135% 5-MeO-DMT in root, 0.092% DMT in leaves.
- Virola sebifera, The bark contains 0.065% to 0.25% alkaloids, most of which are DMT and 5-MeO-DMT.[72]
- Virola venosa, DMT, 5-MeO-DMT[6] in roots, leaves DMT
- Testulea gabonensis: 0.2% 5-MeO-DMT, small quantities of DMT,[56] DMT in bark and root bark, NMTTemplate:Citation needed
Poaceae (Gramineae)
Some Graminae (grass) species contain gramine, which can cause brain damage, other organ damage, central nervous system damage and death in sheep.[73]
- Arundo donax, 0.0057% DMT in dried rhizome, no stem, 0.026% bufotenine, 0.0023% 5-MeO-MMT[74]
- Phalaris aquatica, 0.0007–0.18% Total alkaloids,[75] 0.100% DMT,[76] 0.022% 5-MeO-DMT,[76] 0.005% 5-OH-DMT[76]
- Phalaris arundinacea, 0.0004–0.121% Total alkaloids[75]
- Phalaris brachystachys, aerial parts up to 3% total alkaloids, DMT presentTemplate:Citation needed
- Phalaris coerulescens, Coerulescine and 2-methyl-1,2,3,4-Tetrahydro-β-carboline in rhizome.[77]
- Phragmites australis, DMT, 5-MeO-DMT, bufotenine and gramine in the rhizome.[78]
None of the above alkaloids are said to have been found in Phalaris californica, Phalaris canariensis, Phalaris minor and hybrids of P. arundinacea together with P. aquatica.[75]
- Psychotria carthagenensis, 0.2% average DMT in dried leaves.[79]
- Psychotria colorata, Presence of mu opioid receptor(MOR) agonist and NMDA antagonist: hodgkinsine, psychotridine. Also mentioned in The Encyclopedia of Psychoactive Plants: Ethnopharmacology and Its Applications.[80]
- Psychotria expansa, DMT[63]
- Psychotria forsteriana, DMT[63]
- Psychotria insularum, DMT[63]
- Psychotria poeppigiana,[81] DMT[63]
- Psychotria rostrata, DMT[63]
- Psychotria rufipilis, DMT[63]
- Psychotria viridis, DMT 0.1–0.61% dried mass.[82]
- Dictyoloma incanescens, 5-MeO-DMT in leaves,[71] 0.04% 5-MeO-DMT in bark[56]
- Dutaillyea drupacea, > 0.4% 5-MeO-DMT in leaves[33]
- Dutaillyea oreophila, 5-MeO-DMT[6] in leaves
- Tetradium ruticarpum(syn. Evodia rutaecarpa), 5-MeO-DMT[6] in leaves, fruit and roots
- Limonia acidissima, Traces of DMT;[6] 5-MeO-DMT in stemsTemplate:Citation needed
- Euodia leptococca (formerly Melicope), 0.2% total alkaloids, 0.07% 5-MeO-DMT; 5-MeO-DMT in leaves and stems, also "5-MeO-DMT-Oxide and a beta-carboline"[62]
- Pilocarpus organensis, DMT, 5-MeO-DMT in leaves[85] (Might also contain pilocarpine)
- Vepris ampody, Up to 0.2% DMT in leaves and branches[56]
- Zanthoxylum arborescens, Traces of DMT;[6] DMT in leaves
- Zanthoxylum procerum, DMT in leavesTemplate:Citation needed
- Citrus limon, DMT, N-Methylated tryptamine derivative in leaves[86][87]
- Citrus sinesis,DMT, N-Methylated tryptamine derivative[86][87]
- Citrus bergamia,DMT, N-Methylated tryptamine derivative[86][87]
- Mandarin orange Traces of N-methylated tryptamine derivative in leaf.[88][87]
- Chinotto Tree, N-Methylated tryptamine derivative in leaf[88][87]
- Citrus medica, N-Methylated tryptamine derivative in leaf[88][87]
Phenethylamines



Species, Alkaloid Content (Fresh) – Alkaloid Content (Dried)
- Coryphantha contains various phenethylamine alkaloids including macromerine, coryphanthine, O-methyl-candicine, corypalmine, and N-methyl-corypalmine.[89][90]
- Cylindropuntia echinocarpa (syn. Opuntia echinocarpa), Mescaline 0.01%, DMPEA 0.01%, 4-hydroxy-3-5-dimethoxyphenethylamine 0.01%[91]
- Cylindropuntia spinosior (syn. Opuntia spinosior),[92] Mescaline 0.00004%, 3-methoxytyramine 0.001%, tyramine 0.002%, 3-4-dimethoxyphenethylamine.[91]
- Echinopsis lageniformis (syns Echinopsis scopulicola, Trichocereus bridgesii), Mescaline > 0.025%,[93] also DMPEA < 1%, 3-methoxytyramine < 1%, tyramine < 1%; Mescaline 2%[94]
- Echinopsis macrogona (syn. Trichocereus macrogonus), > 0.01–0.05% Mescaline[95]
- Echinopsis pachanoi (syn. Trichocereus pachanoi), Mescaline 0.006–0.12%, 0.05% Average;[96] Mescaline 0.01%–2.375%[96]
- Echinopsis peruviana (syn. Trichocereus peruvianus), Mescaline 0.0005%–0.12%;[96] Mescaline
- Echinopsis spachiana (syn. Trichocereus spachianus), Mescaline;[91] Mescaline[91]
- Echinopsis tacaquirensis subsp. taquimbalensis (syn. Trichocereus taquimbalensis),[97]Template:Unreliable source? > 0.005–0.025% mescaline[95]
- Echinopsis terscheckii (syn. Trichocereus terscheckii, Trichocereus werdemannianus)[98]Template:Unreliable source? > 0.005–0.025% Mescaline;[95] mescaline 0.01%–2.375%[96]
- Echinopsis valida, 0.025% mescaline[99]Template:Better source needed
- Lophophora williamsii (Peyote), 0.4% Mescaline;[99]Template:Better source needed 3–6% Mescaline[91]
- Opuntia acanthocarpa Mescaline[100]
- Opuntia basilaris Mescaline 0.01%, plus 4-hydroxy-3-5-dimethoxyphenethylamine[91]Template:Better source needed
- Pelecyphora aselliformis, mescaline[99]Template:Better source needed
Beta-carbolines







Beta-carbolines are "reversible" MAO-A inhibitors. They are found in some plants used to make Ayahuasca. In high doses the harmala alkaloids are somewhat hallucinogenic on their own. β-carboline is a benzodiazepine receptor inverse agonist and can therefore have convulsive, anxiogenic and memory enhancing effects.[101]
- Amsonia tabernaemontana, Harman[6]
- Aspidosperma exalatum, Beta-carbolines[102]
- Aspidosperma polyneuron, Beta-carbolines[102]
- Apocynum cannabinum, Harmalol[6]
- Ochrosia nakaiana, Harman[6]
- Pleiocarpa mutica, Beta-carbolines[102]
- Calycanthus occidentalis, Harman;[6] Harmine
- Hammada leptoclada, Harman;[6] Tetrahydroharman, etc.
- Kochia scoparia, Harman;[6] Harmine, etc.
- Guiera senegalensis, Tetrahydroharmine;[6] Harman, etc.
- Carex brevicollis, Harmine, etc.[6]
- Carex parva, Beta-carbolines[102]
- Elaeagnus angustifolia, Harman, etc.[6]
- Elaeagnus commutata, Beta-carbolines[102]
- Elaeagnus hortensis, Tetrahydroharman, etc.[6]
- Elaeagnus orientalis, Tetrahydroharman[6]
- Elaeagnus spinosa, Tetrahydroharman[6]
- Hippophae rhamnoides, Harman, etc.[6]
- Shepherdia argentea, Tetrahydroharmol[6]
- Shepherdia canadensis, Tetrahydroharmol[6]
- Arundo donax, Tetrahydroharman, etc.[6]
- Festuca arundinacea, Harman, etc.[6]
- Lolium perenne, (Perennial Ryegrass), Harman, etc.[6]
- Phalaris aquatica, Beta-carbolines[102]
- Phalaris arundinacea, Beta-carbolines[102]
- Nectandra megapotamica, Beta-carbolines[102]
- Acacia baileyana, Tetrahydroharman[6]
- Acacia complanata, Tetrahydroharman,[6] etc.
- Burkea africana, Harman, etc.[6]
- Desmodium gangeticum, Beta-carbolines[102]
- Desmodium gyrans, Beta-carbolines[102]
- Mucuna pruriens, 6-Methoxyharman, Dihydroharman, Harman[6]
- Petalostylis labicheoides, Tetrahydroharman; MAO's up to 0.5%[63]
- Prosopis nigra, Harmalicin, Harman, etc.[6]
- Shepherdia pulchellum, Beta-carbolines[102]
- Strychnos melinoniana, Beta-carbolines[102]
- Strychnos usambarensis, Harman[102]
- Banisteriopsis argentia, 5-methoxytetrahydroharman, (−)-N(6)-methoxytetrahydroharman, dimethyltryptamine-N(6)-oxide[10]
- Banisteriopsis caapi, Harmine 0.31–0.84%,[103] tetrahydroharmine, telepathine, dihydroshihunine,[104] 5-MeO-DMT in bark[105]
- Banisteriopsis inebrians, Beta-carbolines[102]
- Banisteriopsis lutea, Harmine, telepathine[10]
- Banisteriopsis metallicolor, Harmine, telepathine[10]
- Banisteriopsis muricata Harmine up to 6%, harmaline up to 4%, plus DMT[106]Template:Better source needed
- Diplopterys cabrerana, Beta-carbolines[102]
- Cabi pratensis, Beta-carbolines[102]
- Callaeum antifebrile(syn. Cabi paraensis), Harmine[6]
- Tetrapterys methystica(syn. Tetrapteris methystica), Harmine[107]Template:Failed verification
- Gymnacranthera paniculata, Beta-carbolines[102]
- Horsfieldia superba Beta-carbolines[62]
- Virola cuspidata, 6-Methoxy-Harman[6]
- Virola rufula, Beta-carbolines[102]
- Virola theiodora, Beta-carbolines[102]
- Testulea gabonensis, Beta-carbolines[102]
- Plectocomiopsis geminiflora, Beta-carbolines[102]
- Meconopsis horridula, Beta-carbolines[102]
- Meconopsis napaulensis, Beta-carbolines[102]
- Meconopsis paniculata, Beta-carbolines[102]
- Meconopsis robusta, Beta-carbolines[102]
- Meconopsis rudis, Beta-carbolines[102]
- Papaver rhoeas, Beta-carbolines[102]
- Papaver Bracteatum ~ Tefamine
- Papaver Paeoniflorum ~ Morphine
- Papaver Setigerum ~ Morphine
- Papaver somniferum ~ Morphine

- Passiflora actinia, Harman[6]
- Passiflora alata, Harman[6]
- Passiflora alba, Harman[6]
- Passiflora bryonoides, Harman[6]
- Passiflora caerulea, Harman[6]
- Passiflora capsularis, Harman[6]
- Passiflora decaisneana, Harman[6]
- Passiflora edulis, Harman,[6] 0–7001 ppm[26] in fruit
- Passiflora eichleriana, Harman[6]
- Passiflora foetida, Harman[6]
- Passiflora incarnata (with bee), Harmine, Harmaline, Harman, etc. 0.03%.[108] Alkaloids in rind of fruit 0.25%[108]
- Passiflora quadrangularis, Harman[6]
- Passiflora ruberosa, Harman[6]
- Passiflora subpeltata, Harman[6]
- Passiflora warmingii, Harman[6]
- Calligonum minimum, Beta-carbolines[102]
- Leptactinia densiflora, Tetrahydroharmine,[6] etc.
- Ophiorrhiza japonica, Harman[6]
- Pauridiantha callicarpoides, Harman[6]
- Pauridiantha dewevrei, Harman[6]
- Pauridiantha lyalli, Harman[6]
- Pauridiantha viridiflora, Harman[6]
- Simira klugei, Harman[6]
- Simira rubra, Harman[6]
- Borreria verticillata, Beta-carbolines[102]
- Leptactinia densiflora, Beta-carbolines[102]
- Nauclea diderrichii, Beta-carbolines[102]
- Ophiorrhiza japonica, Beta-carbolines[102]
- Pauridiantha callicarpoides, Beta-carbolines[102]
- Pauridiantha dewevrei, Beta-carbolines[102]
- Pauridiantha yalli, Beta-carbolines[102]
- Pauridiantha viridiflora, Beta-carbolines[102]
- Pavetta lanceolata, Beta-carbolines[102]
- Psychotria carthagenensis, Beta-carbolines[102]
- Psychotria viridis, Beta-carbolines[102]
- Simira klugei, Beta-carbolines[102]
- Simira rubra, Beta-carbolines[102]
- Uncaria attenuata, Beta-carbolines[102]
- Uncaria canescens, Beta-carbolines[102]
- Uncaria orientalis, Beta-carbolines[102]
- Nauclea latifolia, Tramadol
- Tetradium (syn. Evodia) species: Some contain carbolinesTemplate:Citation needed
- Euodia leptococca Beta-carboline[62]
- Araliopsis tabouensis, Beta-carbolines[102]
- Flindersia laevicarpa, Beta-carbolines[102]
- Xanthoxylum rhetsa, Beta-carbolines[102]
- Ailanthus malabarica, Beta-carbolines.[102] (See also Nag Champa)
- Perriera madagascariensis, Beta-carbolines[102]
- Picrasma ailanthoides, Beta-carbolines[102]
- Picrasma crenata, Beta-carbolines[102]
- Picrasma excelsa, Beta-carbolines[102]
- Picrasma javanica, Beta-carbolines[102]
- Vestia foetida, (Syn V. lycioides) Beta-carbolines[102]
- Grewia mollis, Beta-carbolines[102]
- Fagonia cretica, Harman[6]
- Nitraria schoberi, Beta-carbolines[102]
- Peganum harmala, (Syrian Rue), The seeds contain about 2–6% alkaloids, most of which is harmaline.[109]Template:Better source needed Peganum harmala is also an abortifacient.
- Peganum nigellastrum, Harmine[110]
- Tribulus terrestris, Harmine etc.;[6] Harman
- Zygophyllum fabago, Harmine etc.;[6] Harman
Opiates

Opiates are the natural products of many plants, the most famous and historically relevant of which is Papaver somniferum. Opiates are defined as natural products (or their esters and salts that revert to the natural product in the human body), whereas opioids are defined as semi-synthetic or fully synthetic compounds that trigger the Opioid receptor of the mu sub-type. Other opiate receptors, such as kappa- and delta-opiate receptors are part of this system but do not cause the characteristic behavioral depression and analgesia which is mostly mediated through the mu-opiate receptor.
An opiate, in classical pharmacology, is a substance derived from opium. In more modern usage, the term opioid is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain (including antagonists). Opiates are alkaloid compounds naturally found in the Papaver somniferum plant (opium poppy). The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions with evidence of opiate trade and use for pain relief as early as the eighth century AD. Opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.
In 2014, between 13 and 20 million people used opiates recreationally (0.3% to 0.4% of the global population between the ages of 15 and 65). According to the CDC, from this population, there were 47,000 deaths, with a total of 500,000 deaths from 2000 to 2014. In 2016, the World Health Organization reported that 27 million people suffer from Opioid use disorder. They also reported that in 2015, 450,000 people died as a result of drug use, with between a third and a half of that number being attributed to opioids.

The plant contains a latex that thickens into opium when it is dried. Opium contains approximately 40 alkaloids, which are summarized as opium alkaloids.[6] The main psychoactive alkaloids are:
- Morphine: 3 to 20% in opium[6]
- Codeine 0.1 to 4% in opium[6]
- Thebaine 0.1 to 4% in opium[6]
- Noscapine 1 to 11% in opium[6]
- Oripavine
Laurelia novae-zelandiae ~ pukateine
- Mitragynine: Approx. 0.33% in dried leaves[6]
- 7-Hydroxymitragynine[111]
- Mitragynine pseudoindoxylTemplate:Citation needed
- Akuammicine[112]
- Pericine[113] It may also have convulsant effects.[114]

Plants containing other psychoactive substances
Template:More citations needed
| Substance(s) | Plant | Comments |
|---|---|---|
 |
 |
Toxic.Template:Citation needed |
 |
Alchornea floribunda | α2-adrenergic receptor antagonist.Template:Citation needed |
  |
 |
GABA uptake inhibitor,[118][119] stimulant.[120] |
 |

|
Used by Chinese residents of Mexico during the early 20th century as a legal substitute for opium and currently smoked as a marijuana substitute.Template:Citation needed |
 |

Argyreia nervosa (Hawaiian Baby Woodrose) |
Seeds contain ergine (also known as LSA), often 50-150X the amounts found in Ipomoea violacea. LSA is a hallucinogen.[121] |
 |
 |
Also called "wormwood". GABA receptor antagonist.[122] |
| Quinoline & Aporphine alkaloids |  Asimina triloba (Paw Paw) |
Identical alkaloid to morphine.[123] |
   |
 |
Commonly known as 'deadly nightshade'. An anticholinergic deliriant.[124] |
   |
 |
Commonly known as 'angel's trumpets'. An anticholinergic deliriant.[124] |
   Indole alkaloids (harmine, manacine, brunfelsamidine), Tropane alkaloids (scopolamine) |

|
Known to cause delirium, sustained mental confusion, and possible blindness.[125] |
| Unknown | File:Calea zacatechichi cutting.jpg | Produces vivid dreams after smoking. It is also employed by the Chontal people as a medicinal herb against gastrointestinal disorders, and is used as an appetizer, cathartic anti-dysentery remedy, and as a fever-reducing agent. Its psychedelic properties do not become apparent until the user is asleep. Reports describe rituals that involve drinking it as a tea to induce divinatory or lucid dreams due to its properties as an oneirogen.[126] |
 |
 |
Tea leaves, tea, native to Asia.Template:Citation needed |
 |

|
Khat, commonly chewed, produces a stimulant effect.[127] |
 |
 |
Catharanthus roseus is (perhaps unpleasantly) "hallucinogenic."[128][129]Template:Unreliable source? |
| Unknown |  |
Commonly referred to as 'night-blooming jasmine', 'lady of the night', and 'poisonberry'. It has an unknown mechanism of action.Template:Citation needed |
 |
 |
Coffee beans, coffee, native to Africa.[130] |
 |
 |
Cola or kola nut, traditional additive to cola, native to Africa.Template:Citation needed |
| (Unknown) | 
|
Unknown |
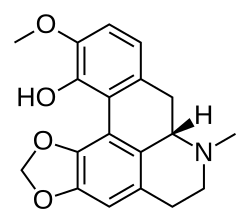
|

Corydalis solida, cava |
Bulbocapnine, Nantenine, Tetrahydropalmatine |
  Tropane alkaloids (Scopolamine, Atropine) |

|
Also known as 'thorn apple', 'devil's trumpets', 'loco weed', and 'Jimson weed'. Scopolamine and Atropine are both anticholinergics[131][132] which produce hallucinogenic and deliriant effects. It has an extensive history of being used recreationally.[133] |
 |
 |
Nicotine-like effects. partial agonist of nicotinic acetylcholine receptors (nAChRs).[134] |
| Unknown |  |
Causes visions.[135] |
 |
 |
Pituri |
| Unknown |  |
African dream herb.Template:Citation needed |
 |
 |
Ephedra |
 |
 |
Coca. Widely used illegal stimulant, produces hallucination in overdose, native to South America.Template:Citation needed |
| Unknown |  |
Nerve or mosaic plant, said to produce vision of eyeballs |
 |
Galbulimima belgraveana | Galbulimima belgraveana is rich in alkaloids and twenty-eight alkaloids have been isolated including himbacine.Template:Citation needed |
 |

|
Hallucinogenic effects.[136] |
 Possibly CryogenineTemplate:Citation needed |
Heimia myrtifolia | Auditory |
 Possibly CryogenineTemplate:Citation needed |
 |
Auditory[137]Template:Better source needed |
  |
 |
Star of Bethlehem |
 |
 |
Saint John's wort |
| Tropane alkaloids |  |
Henbane |
  Caffeine, Theobromine, Dimethylxanthines |
 |
Ilex guayusa is used as an additive to some versions of Ayahuasca. According to the Ecuadorian indigenous, it is also slightly hallucinogenic on its own, when drunk in high enough quantities.Template:Citation needed |
 |
 |
Ergine in seeds; up to 0.12% total[138]Template:Better source needed Produces psychedelic effects. |
| Unknown |  |
Unknown |
| Lactucarium | 
|
Lactucarium |
|
 |
Lagochilin is thought to be responsible for the sedative, hypotensive and hemostatic effects of this plant.Template:Citation needed |
 |

|
Pukateine |
| Unknown |  |
Rollinia mucosa is said to be a narcotic.[123] |
 |

|
Both leaves and flowers (where most concentrated) contain Leonurine. (Effects reminiscent of marijuana)Template:Citation needed |
 |
 Leucas aspera |
Nicotine |
 |

|
Both leaves and flowers (where most concentrated) contain Leonurine and several compounds. (Effects reminiscent of marijuana)Template:Citation needed[140] |
 |
 |
Indian tobacco |
| Unknown | 
|
[6] |
   |
 |
Mandrake has deliriant and anticholinergic properties.[124] |
 |
 Some Mirabilis spp. |
Possibly contains ergineTemplate:Citation needed, a hallucinogen. |
  |
 |
Usually referred to as kratom. Has opioid-like and stimulant properties.[141] |
 |
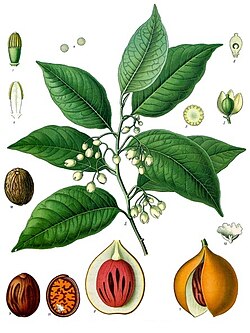 |
Nutmeg |
 |
 |
Sacred lotus |
 |
 |
Catnip |
 |
 |
Tobacco. Can cause hallucinations in very large doses.Template:Citation needed |
 |

|
Blue lotus or lily. Recent studies have shown Nymphaea caerulea to have psychedelic properties, and may have been used as a sacrament in ancient Egypt and certain ancient South American cultures. Dosages of 5 to 10 grams of the flowers induces slight stimulation, a shift in thought processes, enhanced visual perception, and mild closed-eye visuals.[142] Nymphaea caerulea is unrelated to Nelumbo nucifera the Sacred Lotus, with Nymphaea in the Nymphales, one of the oldest and most basal linegages of flowering plants and with Nelumbo in Proteales one of the core eudicots. Their morphological similarties being entirely convergent evolution, however they apparently have convergently evolved similar biochemistry. Both Nymphaea caerulea and Nelumbo nucifera contain the alkaloids nuciferine and apomorphine, which have been recently isolated by independent labs.Template:Citation needed
These psychoactive effects make Nymphaea caerulea a likely candidate (among several) for the lotus plant eaten by the mythical Lotophagi in Homer's Odyssey. Used in aromatherapy, Nymphaea caerulea is purported to have a "divine" essence, bringing euphoria, heightened awareness and tranquility.Template:Citation needed Other sources cite anti-spasmodic and sedative, purifying and calming properties. |
 |
 |
Ginseng |
 |
 |
Opium. Widely used analgesic, native to the Old World.[143] |
| Unknown |  |
Narcotic and toxic when the root is consumed.[123] |
 |
 |
α2-adrenergic receptor antagonist.Template:Citation needed |
| Unknown |  |
Indian warrior |
 |

|
An anxiolytic[144] and hypnotic.[145] Often advertised as a 'healthier' alternative to alcohol.Template:Citation needed |
 |
 |
Seeds contain ergine, lysergol, and turbicoryn; lysergic acid alkaloids up to 0.03%[146]Template:Better source needed Has psychedelic properties. |
 |

|
Salvinorin A, 0.89–3.87 mg/g, also Salvinorin B and Salvinorin C[147]Template:Unreliable source? |
 Mesembrine |
 |
Kanna[148][149] |
 |
 |
Known commonly as 'skullcaps'. Baicalein is a positive allosteric modulator of GABAA receptor.[150] |
| Unknown | 
|
S. brasiliensis poisoning is described as very similar to that of Cestrum laevigatum; a species used to induce hallucinations by the Krahô tribe for spiritual purposes.[151] [152] |
| Unknown | 
|
Produces vivid dreams after smoking.[153] |
| Unknown | 
|
Anethole, Chavicol, Coumarin, Estragole, Isorhamnetin, Methyleugenol, Quercitin |
 |

|
Ibogaine in root bark. Produces psychedelic and a dissociative effects.[154][155] |
 |
Tabernanthe orientalis |
Ibogaine in root leaves. Produces psychedelic and a dissociative effects.[154][155] |
  |

|
Is a psychedelic and a dissociative.[155] |
 |
Tabernanthe pubescens |
Is a psychedelic and a dissociative. Contains ibogaine and similar alkaloids.[154][155] |
 |
 Tabernaemontana sp. |
Is a psychedelic and a dissociative.[154][155] |
 |
 |
Cocoa or cacao bean, chocolate, native to the Americas |
 |

|
Exhibits psychedelic and dissociative effects. Contains ibogaine, coronaridine, voacangine, apparicine, conoflorine, and 19-epi-voacangarine.[156]Template:Better source needed[157] |
 |
 |
Possible sedative and anxiolytic effects. Valerenic acid is GABAA receptor positive allosteric modulator,[158] and a 5-HT5A receptor partial agonist.[159] |
 |
 |
Vincamine.[160] |
 |
 |
Voacangine is similar in structure to ibogaine. It inhibits AChE.[161][162] |
 |
 |
Also contains phenanthrenes and dendrobine related alkaloids. |
  |
 |
Zornia latifolia is sometimes combined with synthetic cannabis. It may produce similar effects to cannabis.[164][165] It is nicknamed Maconha brava because locals use it as a cannabis substitute.Template:Citation needed |
See also
- Aztec entheogenic complex
- Entheogenic drugs and the archaeological record
- God in a Pill?
- Hallucinogenic fish
- Hallucinogenic plants in Chinese herbals
- List of Acacia species known to contain psychoactive alkaloids
- List of entheogenic/hallucinogenic species
- List of plants used for smoking
- List of poisonous plants
- List of psychoactive plants, fungi, and animals
- Louisiana State Act 159
- N,N-Dimethyltryptamine
- Psilocybin mushrooms
- Psychoactive cactus
- Psychoactive plant
Notes
References
Bibliography
External links
- Descriptions of psychoactive Cacti. Lycaeum Visionary Cactus Guide
- Erowid Tryptamine FAQ – More Plants Containing Tryptamines
- John Stephen Glasby, Dictionary of Plants Containing Secondary Metabolites, Published by CRC Press
- Golden Guide to Hallucinogenic Plants
- Hallucinogens on the Internet: A Vast New Source of Underground Drug Information John H. Halpern, M.D. and Harrison G. Pope, Jr., M.D.
- Chemical Investigations of the Alkaloids from the Plants of the Family Elaeocarpaceae – Peter L. Katavic, Chemical Investigations of the Alkaloids From the Plants Of The Family Elaeocarpaceae, School of Science/Natural Product Discovery (NPD), Faculty of Science, Griffith University
- Alexander T. Shulgin, Psychotomimetic Drugs: Structure-Activity Relationships
- UNODC The plant kingdom and hallucinogens (part II)
- UNODC The plant kingdom and hallucinogens (part III)
- Virola – Dried Herbarium Specimens
- Virola Species Pictures – USGS
- Desmanthus illinoensis – USDA
- Psychedelic Reader (Google Books)
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 Template:Cite web
- ↑ Template:Cite web
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 6.16 6.17 6.18 6.19 6.20 6.21 6.22 6.23 6.24 6.25 6.26 6.27 6.28 6.29 6.30 6.31 6.32 6.33 6.34 6.35 6.36 6.37 6.38 6.39 6.40 6.41 6.42 6.43 6.44 6.45 6.46 6.47 6.48 6.49 6.50 6.51 6.52 6.53 6.54 6.55 6.56 6.57 6.58 6.59 6.60 6.61 6.62 6.63 6.64 6.65 6.66 6.67 6.68 6.69 6.70 6.71 6.72 6.73 6.74 6.75 6.76 6.77 6.78 6.79 6.80 6.81 6.82 6.83 6.84 6.85 6.86 6.87 6.88 Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 9.14 Template:Cite web
- ↑ 10.0 10.1 10.2 10.3 Template:Cite book
- ↑ Nutritive value assessment of the tropical shrub legume Acacia angustissima: anti-nutritional compounds and in vitro digestibility. Personal Authors: McSweeney, C. S., Krause, D. O., Palmer, B., Gough, J., Conlan, L. L., Hegarty, M. P.Author Affiliation: CSIRO Livestock Industries, Long Pocket Laboratories, 120 Meiers Road, Indooroopilly, Qld 4068, Australia. Document Title: Animal Feed Science and Technology, 2005 (Vol. 121) (No. 1/2) 175–190
- ↑ Template:Cite web
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 Template:Cite web
- ↑ Template:Cite web
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 Template:Cite book
- ↑ 16.0 16.1 16.2 16.3 Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ NMR spectral assignments of a new chlorotryptamine alkaloid and its analogues from Acacia confusa Malcolm S. Buchanan, Anthony R. Carroll, David Pass, Ronald J. Quinn Magnetic Resonance in Chemistry Volume 45, Issue 4, pp. 359–361. John Wiley & Sons, Ltd.
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ 26.0 26.1 26.2 Template:Cite web
- ↑ 27.0 27.1 Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ 30.0 30.1 30.2 Template:Cite book
- ↑ Template:Cite web
- ↑ Voogelbreinder, S. "Garden Of Eden" 2009
- ↑ 33.0 33.1 33.2 Template:Cite web
- ↑ Template:Cite web
- ↑ extentech.sheetster.comTemplate:Dead link
- ↑ S. Voogelbreinder "Garden Of Eden" 2009
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Plants Containing DMT (German) Template:Webarchive
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ 44.0 44.1 44.2 44.3 44.4 44.5 Template:Cite web
- ↑ 45.0 45.1 45.2 45.3 45.4 45.5 45.6 45.7 Dr. Duke's Template:Webarchive Phytochemical and Ethnobotanical Databases
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Dr. Duke's Template:Webarchive Phytochemical and Ethnobotanical Databases
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ 51.0 51.1 Template:Cite web
- ↑ Template:Cite book
- ↑ Template:GRIN
- ↑ 54.0 54.1 54.2 Template:Cite web
- ↑ Template:Cite book
- ↑ 56.0 56.1 56.2 56.3 56.4 56.5 56.6 56.7 56.8 Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite book
- ↑ 62.0 62.1 62.2 62.3 62.4 Template:Cite web
- ↑ 63.0 63.1 63.2 63.3 63.4 63.5 63.6 63.7 63.8 [1]Template:Dead link
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite webTemplate:Dead link
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ 71.0 71.1 Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite book
- ↑ Template:Cite web
- ↑ 75.0 75.1 75.2 Template:Cite web
- ↑ 76.0 76.1 76.2 Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 86.0 86.1 86.2 Template:Cite web
- ↑ 87.0 87.1 87.2 87.3 87.4 87.5 Template:Cite web
- ↑ 88.0 88.1 88.2 Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 91.0 91.1 91.2 91.3 91.4 91.5 Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ 95.0 95.1 95.2 Template:Cite web
- ↑ 96.0 96.1 96.2 96.3 Forbidden Fruit Archives Template:Webarchive
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ 99.0 99.1 99.2 Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ 102.00 102.01 102.02 102.03 102.04 102.05 102.06 102.07 102.08 102.09 102.10 102.11 102.12 102.13 102.14 102.15 102.16 102.17 102.18 102.19 102.20 102.21 102.22 102.23 102.24 102.25 102.26 102.27 102.28 102.29 102.30 102.31 102.32 102.33 102.34 102.35 102.36 102.37 102.38 102.39 102.40 102.41 102.42 102.43 102.44 102.45 102.46 102.47 102.48 102.49 102.50 102.51 102.52 102.53 102.54 102.55 Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ 108.0 108.1 Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 123.0 123.1 123.2 Template:Cite journal
- ↑ 124.0 124.1 124.2 Template:Cite book
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite web
- ↑ Template:Cite news
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Schultes, Richard Evans, Iconography of New World Plant Hallucinogens. p. 101
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 154.0 154.1 154.2 154.3 Template:Cite web
- ↑ 155.0 155.1 155.2 155.3 155.4 Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal