Methane (data page)
Template:Short description This page provides supplementary chemical data on methane.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions.[1]
Structure and properties
| Template:Chembox header | Structure and properties | |
|---|---|
| Index of refraction, nD | 1.000444[2] |
| Dielectric constant, εr | 1.6761 ε0 at −182 °C[3] 1.0008181 ε0 at −20 °C[4] |
| Bond strength | ? |
| Bond length | 0.10870 nm [5] |
| Bond angle | 109.5° |
| Magnetic susceptibility | −17.4Template:E cm3/mol[6] |
Thermodynamic properties
| Template:Chembox header | Phase behavior | |
|---|---|
| Triple point | 90.67 K (−182.48 °C), 0.117 bar[7] |
| Critical point | 190.6 K (−82.6 °C), 46.0 bar[7] |
| Std enthalpy change of fusion, ΔfusH |
1.1 kJ/mol |
| Std entropy change of fusion, ΔfusS |
12.1 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
8.17 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Template:Chembox header | Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Template:Chembox header | Liquid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Template:Chembox header | Gas properties | |
| Std enthalpy change of formation, ΔfH |
−74.6 kJ/mol[8] |
| Standard molar entropy, S |
186.3 J/(mol K)[8] |
| Enthalpy of combustion ΔcH |
−802 kJ/mol[9] |
| Heat capacity, cp | 35.7 J/(mol K)[8] |
| van der Waals' constants[10] | a = 228.29 L2 kPa/mol2 b = 0.04278 L/mol |
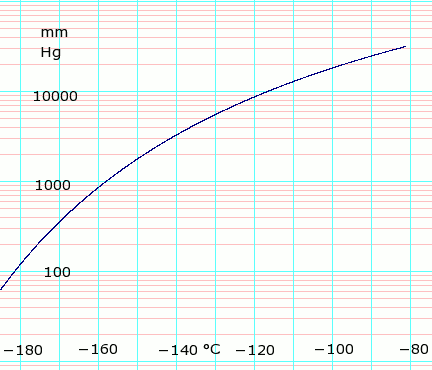
Vapor pressure of liquid
| Template:Chembox header | P (mm Hg) | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 |
| Template:Chembox header | T (°C) | −205.9(s) | −195.5(s) | −187.7(s) | −181.4 | −168.8 | −161.5 | −152.3 | −138.3 | −124.8 | −108.5 | −86.3 | — |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed. Annotation "(s)" indicates equilibrium temperature of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid. Note that these are all negative temperature values.
Spectral data

| Template:Chembox header | UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| Template:Chembox header | IR | |
| Major absorption bands | 3019, 2917, 1534, 1306 cm−1[11] |
| Template:Chembox header | NMR | |
| Proton NMR | |
| Carbon-13 NMR | −2.3 ppm[12] |
| Other NMR data | |
| Template:Chembox header | MS | |
| Masses of main fragments |
Template:Chemical data page general note
References
- ↑ Material Safety Datasheet . Iowa State
- ↑ Haynes, p. 10.256
- ↑ Haynes, p. 6.212
- ↑ Haynes, p. 6.221
- ↑ Haynes, p. 9.45
- ↑ Haynes, p. 3.578
- ↑ 7.0 7.1 Haynes, p. 6.175
- ↑ 8.0 8.1 8.2 Haynes, p. 5.26
- ↑ Haynes, p. 5.67
- ↑ Lange's Handbook of Chemistry, 10th ed, pp. 1522–1524.
- ↑ Haynes, p. 9.106
- ↑ Haynes, p. 8.67