Dichloromethane (data page)
Template:Short description Template:Use dmy dates Please find below supplementary chemical data about dichloromethane.
MSDS sheets
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties
| Template:Chembox header | Structure and properties | |
|---|---|
| Index of refraction,[1] nD | 1.4242 |
| Abbe number | ? |
| Dielectric constant,[2] εr | 9.08 ε0 at 20 °C |
| Dipole moment,[3] | 1.62 D |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension[4] | 26.52 dyn/cm at 20 °C |
| Viscosity[5] | 0.449 mPa·s at 15 °C 0.393 mPa·s at 30 °C |
Thermodynamic properties
| Template:Chembox header | Phase behavior | |
|---|---|
| Triple point | ? K (? °C), ? Pa |
| Critical point[6] | 510 K (237 °C), 6100 kPa |
| Std enthalpy change of fusion, ΔfusH |
+6.160 kJ/mol |
| Std entropy change of fusion, ΔfusS |
? J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
28.6 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
91.43 J/(mol·K) |
| Template:Chembox header | Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Template:Chembox header | Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−124.3 kJ/mol |
| Standard molar entropy, S |
174.5 J/(mol K) |
| Heat capacity, cp | 102.3 J/(mol K) |
| Template:Chembox header | Gas properties | |
| Std enthalpy change of formation, ΔfH |
−95.52 kJ/mol |
| Standard molar entropy, S |
270.28 J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| van der Waals' constants[7] | a = 1244 L2 kPa/mol2 b = 0.08689 liter per mole |
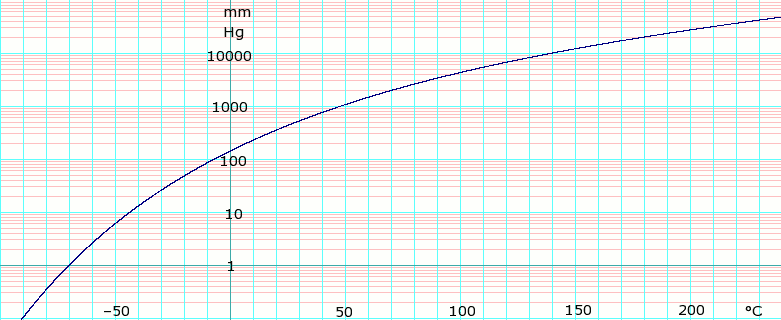
Vapor pressure of liquid
| Template:Chembox header | P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 |
| Template:Chembox header | T in °C | –70.0 | –43.3 | –22.3 | –6.3 | 24.1 | 40.7 |
Table data obtained from CRC Handbook of Chemistry and Physics 47th ed.
Spectral data
| UV-Vis | |
|---|---|
| Spectrum | ? |
| Lambda-max | ? nm |
| Log Ε | ? |
| Template:Chembox header colspan="2" | IR | |
| Spectrum | NIST |
| Major absorption bands | ? cm−1 |
| Template:Chembox header colspan="2" | NMR | |
| Proton NMR | δ CDCl3 5.30 (s, 2H) |
| Carbon-13 NMR | δ CDCl3 53.5 |
| Other NMR data | ? |
| MS | |
| Masses of main fragments |
? |
Structure and properties data
| Structure and properties | |
|---|---|
| Index of refraction | 1.424 |
| Dielectric constant | 8.93 |
| Viscosity | 0.44 cP at 20 °C |
Template:Chemical data page general note
References
- NIST website
- G. W. C. Kaye and T. H. Laby, Tables of Physical & Chemical Constants at National Physical Laboratory
- Heat capacity
- ↑ Template:Cite web
- ↑ CRC Handbook of Chemistry and Physics, 47th ed. p E-54
- ↑ The Microwave Spectra, Structure, Dipole Moment, and Chlorine Nuclear Quadrupole Coupling Constants of Methylene Chloride Rollie J. Myers and William D. Gwinn, J. Chem. Phys. 20, 1420 (1952); doi:10.1063/1.1700773
- ↑ CRC Handbook of Chemistry and Physics, 47th ed. pp F-28 - F-30
- ↑ CRC Handbook of Chemistry and Physics, 47th ed. pp F-33 - F-38
- ↑ 6.0 6.1 Template:Cite web
- ↑ Template:Cite web