Isotopes of beryllium
Template:Short description Template:More citations needed Template:Infobox beryllium isotopes
Beryllium (4Be) has 11 known isotopes and 3 known isomers, but only one of these isotopes (Template:SimpleNuclide) is stable and a primordial nuclide. As such, beryllium is considered a monoisotopic element. It is also a mononuclidic element, because its other isotopes have such short half-lives that none are primordial and their abundance is very low (standard atomic weight is Template:Val). Beryllium is unique as being the only monoisotopic element with both an even number of protons and an odd number of neutrons. There are 25 other monoisotopic elements but all have odd atomic numbers, and even numbers of neutrons.
Of the 10 radioisotopes of beryllium, the most stable are Template:SimpleNuclide with a half-life of Template:Val million yearsTemplate:Refn and Template:SimpleNuclide with a half-life of Template:Val. All other radioisotopes have half-lives under Template:Val, most under Template:Val. The least stable isotope is Template:SimpleNuclide, with a half-life of Template:Val.
The 1:1 neutron–proton ratio seen in stable isotopes of many light elements (up to oxygen, and in elements with even atomic number up to calcium) is prevented in beryllium by the extreme instability of Template:SimpleNuclide toward alpha decay, which is favored due to the extremely tight binding of [[helium#Related stability of the helium-4 nucleus and electron shell|Template:SimpleNuclide]] nuclei. The half-life for the decay of Template:SimpleNuclide is only Template:Val.
Beryllium is prevented from having a stable isotope with 4 protons and 6 neutrons by the very lopsided neutron–proton ratio for such a light element. Nevertheless, this isotope, [[beryllium-10|Template:SimpleNuclide]], has a half-life of Template:Val million years,Template:Refn which indicates unusual stability for a light isotope with such a large neutron/proton imbalance. Other possible beryllium isotopes have even more severe mismatches in neutron and proton number, and thus are even less stable.
Most Template:SimpleNuclide in the universe is thought to be formed by cosmic ray nucleosynthesis from cosmic ray spallation in the period between the Big Bang and the formation of the Solar System. The isotopes Template:SimpleNuclide, with a half-life of Template:Val, and Template:SimpleNuclide are both cosmogenic nuclides because they are made on a recent timescale in the Solar System by spallation,[1] like [[carbon-14|Template:SimpleNuclide]].
List of isotopes
Template:Isotopes table
|-id=Beryllium-5
| Template:SimpleNuclide[n 1]
|4
|1
| Template:Val#
|
| p ?[n 2]
| Template:SimpleNuclide ?
| (1/2+)#
|
|-id=Beryllium-6
| Template:SimpleNuclide
|4
|2
| Template:Val
| Template:Val
[[[:Template:Val]]]
| 2p
| Template:SimpleNuclide
| 0+
|
|-
| Template:SimpleNuclide[n 3]
|4
|3
| Template:Val
| Template:Val
| ε
| Template:SimpleNuclide
| 3/2−
| Trace[n 4]
|-
| [[Beryllium-8|Template:SimpleNuclide]][n 5]
|4
|4
| Template:Val
| Template:Val
[[[:Template:Val]]]
| α[n 6]
| Template:SimpleNuclide
| 0+
|
|-id=Beryllium-8m
| style="text-indent:1em" | Template:SimpleNuclide
| colspan="3" style="text-indent:2em" | Template:Val
|
| α
| Template:SimpleNuclide
| 2+
|
|-id=Beryllium-9
| Template:SimpleNuclide
|4
|5
| Template:Val
| colspan=3 align=center|Stable
| 3/2−
| 1
|-id=Beryllium-9m
| style="text-indent:1em" | Template:SimpleNuclide
| colspan="3" style="text-indent:2em" | Template:Val
| Template:Val
[[[:Template:Val]]]
|
|
| 3/2−
|
|-
| [[Beryllium-10|Template:SimpleNuclide]]
|4
|6
| Template:Val
| Template:ValTemplate:Refn
| β−
| Template:SimpleNuclide
| 0+
| Trace[n 4]
|-id=Beryllium-11
| rowspan=3|Template:SimpleNuclide[n 7]
| rowspan=3|4
| rowspan=3|7
| rowspan=3|Template:Val
| rowspan=3|Template:Val
| β− (Template:Val)
| Template:SimpleNuclide
| rowspan=3|1/2+
| rowspan=3|
|-
| β−α (Template:Val)
| Template:SimpleNuclide
|-
| β−p (Template:Val)
| Template:SimpleNuclide
|-id=Beryllium-11m
| style="text-indent:1em" | Template:SimpleNuclide
| colspan="3" style="text-indent:2em" | Template:Val
| Template:Val
[[[:Template:Val]]]
| IT ?[n 2]
| Template:SimpleNuclide ?
| 3/2−
|
|-id=Beryllium-12
| rowspan=2|Template:SimpleNuclide
| rowspan=2|4
| rowspan=2|8
| rowspan=2|Template:Val
| rowspan=2|Template:Val
| β− (Template:Val)
| Template:SimpleNuclide
| rowspan=2|0+
| rowspan=2|
|-
| β−n (Template:Val)
| Template:SimpleNuclide
|-id=Beryllium-12m
| style="text-indent:1em" | Template:SimpleNuclide
| colspan="3" style="text-indent:2em" | Template:Val
| Template:Val
| IT
| Template:SimpleNuclide
| 0+
|
|-id=Beryllium-13
| Template:SimpleNuclide
|4
|9
| Template:Val
| Template:Val
| n ?[n 2]
| Template:SimpleNuclide ?
| (1/2−)
|
|-id=Beryllium-13m
| style="text-indent:1em" | Template:SimpleNuclide
| colspan="3" style="text-indent:2em" | Template:Val
|
|
|
| (5/2+)
|
|-id=Beryllium-14
| rowspan=5|Template:SimpleNuclide[n 8]
| rowspan=5|4
| rowspan=5|10
| rowspan=5|Template:Val
| rowspan=5|Template:Val
| β−n (Template:Val)
| Template:SimpleNuclide
| rowspan=5|0+
| rowspan=5|
|-
| β− (> Template:Val)
| Template:SimpleNuclide
|-
| β−2n (Template:Val)
| Template:SimpleNuclide
|-
| β−t (Template:Val)
| Template:SimpleNuclide
|-
| β−α (< Template:Val)
| Template:SimpleNuclide
|-id=Beryllium-14m
| style="text-indent:1em" | Template:SimpleNuclide
| colspan="3" style="text-indent:2em" | Template:Val
|
|
|
| (2+)
|
|-id=Beryllium-15
| Template:SimpleNuclide
|4
|11
| Template:Val
| Template:Val
| n
| Template:SimpleNuclide
| (5/2+)
|
|-id=Beryllium-16
| Template:SimpleNuclide
|4
|12
| Template:Val
| Template:Val
[[[:Template:Val]]]
|2n
|Template:SimpleNuclide
| 0+
|
Template:Isotopes table/footer
Beryllium-7
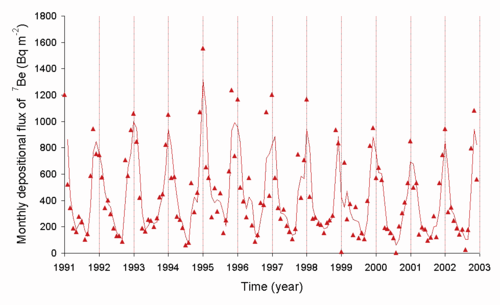
Beryllium-7 is an isotope with a half-life of 53.3 days that is generated naturally as a cosmogenic nuclide.[1] The rate at which the short-lived Template:SimpleNuclide is transferred from the air to the ground is controlled in part by the weather. Template:SimpleNuclide decay in the Sun is one of the sources of solar neutrinos, and the first type ever detected using the Homestake experiment. Presence of Template:SimpleNuclide in sediments is often used to establish that they are fresh, i.e. less than about 3–4 months in age, or about two half-lives of Template:SimpleNuclide.[2]
Beryllium-10

Beryllium-10 has a half-life of Template:Val, and decays by beta decay to stable boron-10 with a maximum energy of 556.2 keV.[3][4] It is formed in the Earth's atmosphere mainly by cosmic ray spallation of nitrogen and oxygen.[5][6][7] 10Be and its daughter product have been used to examine soil erosion, soil formation from regolith, the development of lateritic soils and the age of ice cores.[8] 10Be is a significant isotope used as a proxy data measure for cosmogenic nuclides to characterize solar and extra-solar attributes of the past from terrestrial samples.[9]
Decay chains
Most isotopes of beryllium within the proton/neutron drip lines decay via beta decay and/or a combination of beta decay and alpha decay or neutron emission. However, Template:SimpleNuclide decays only via electron capture, a phenomenon to which its unusually long half-life may be attributed. Notably, its half-life can be artificially lowered by 0.83% via endohedral enclosure (7Be@C60).[10] Also anomalous is Template:SimpleNuclide, which decays via alpha decay to Template:SimpleNuclide. This alpha decay is often considered fission, which would be able to account for its extremely short half-life.
Notes
References
Template:Navbox element isotopes Template:Authority control
Cite error: <ref> tags exist for a group named "n", but no corresponding <references group="n"/> tag was found